Abstract
Introduction Chimeric antigen receptor T-cell therapy (CAR T) represents a revolution in cancer therapeutics for patients with relapsed, refractory, or recurrent malignancies; however, there is still a lack of longitudinal data on real-world patient experiences, particularly regarding education, treatment expectations, patient burden, and preferred patient reported outcomes (PROs) assessing quality of life (QoL). This non-interventional study aims to identify treatment priorities, values, goals, educational needs, and care experiences of individuals considering and participating in CAR T clinical trials, with the goal to generate knowledge to help optimize future clinical trial operations, communications, and QoL assessments.
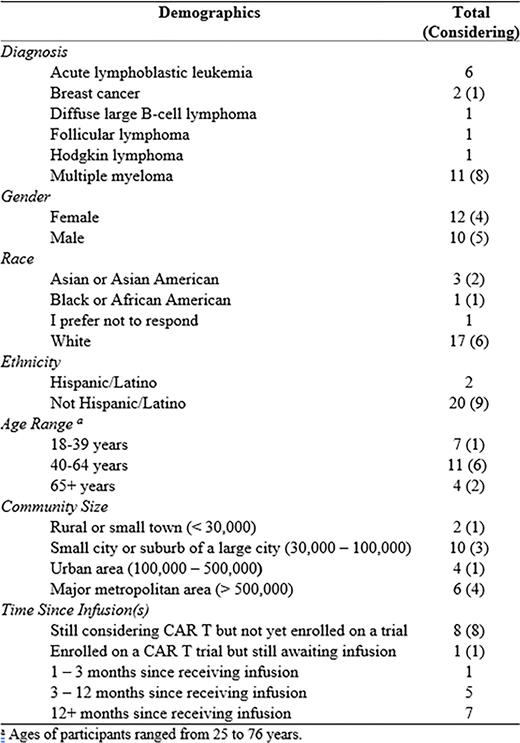
Methods Individuals with hematologic or solid malignancies were recruited through the Gryt Health Cancer Community and social media to enroll in this IRB-approved, mixed-methods study. Participants were recruited into two groups: individuals considering and eligible for a CAR T clinical trial (Group 1), and individuals who participated in a CAR T clinical trial (Group 2). This initial analysis includes: a 1:1 semi-structured interview obtaining individual perceptions of/experiences with CAR T; 1 of 2 patient advisory boards (PABs) utilizing both quantitative questions and qualitative discussion to garner patient insight on CAR T; and 3 of 4 surveys exploring key domains of QoL through assessment of relevance of an existing patient reported outcome measure (PROM) in comparison to a proposed CAR T-specific PROM.
Results Twenty-two participants were enrolled. All participants completed at least the initial 1:1 interview. Both groups perceived freedom from standard/maintenance therapies and current level of pain as an expected/experienced benefit of CAR T. Group 1's initial impressions of CAR T were generally positive, while many in Group 2 reported initial hesitation due to perceived similarity to other treatment. Both groups noted their experiences and/or perceptions of stem cell transplant (SCT) influenced their thoughts on CAR T. Group 2 participants noted that CAR T was far less difficult than SCT, despite their initial fears of the therapies' similarities. Group 1 participants were optimistic about avoiding toxicities associated with SCT by pursuing CAR T instead.
In Survey 1, participants completed the PROMIS-29 v2.0 to measure the perceived relevance of the instrument. Comparison of reflection responses showed that Group 2 participants found the questions less relevant than those in Group 1. Overall, participants indicated that the current PROMIS-29 v2.0, while somewhat relevant, has gaps to be addressed, including assessment of potential long-term side effects and their impact on QoL at each phase of CAR T (pre-infusion, acute post infusion period, and long-term follow-up). In Survey 2, both groups identified the following QoL aspects to be addressed by a CAR T-specific PROM: exercise, ability to carry out daily responsibilities, memory, and sleep. In Survey 3, both groups reviewed a proposed CAR T-specific PROM and felt it would be useful assessing their treatment concerns and QoL. Most participants indicated the PROM would be a useful tool for communication with their care team, and access to results over time would be useful for reflection and may impact willingness to complete the PROM.
PAB questions highlighted aspects of CAR T education in need of improvement. For both groups, CAR T education most commonly began with the patient's care team, with some solely relying on their doctors' suggestions and others conducting their own additional research. When asked about preferable CAR T educational formats, both groups most often answered a 1:1 discussion with a member of their care team. Noted second most often was the ability to speak with patients who had received CAR T already. Both groups had varying understanding of the science, the mechanism of action, and the manufacturing of CAR T.
Conclusion This study reflects on the heterogeneity of experiences of those who are considering or have undergone CAR T-cell therapy, providing meaningful information regarding the educational needs and lived experiences throughout the CAR T treatment experience. Ongoing analysis will continue to identify perceived burdens of CAR T and trial participation, assess lived experiences, and assess the proposed CAR T-specific PROM to be tested in future studies.
Disclosures
Byrd:Syneos Health: Research Funding. Flora:Syneos Health: Research Funding. Schneider:Contract Research Organization: Research Funding; Syneos Health: Research Funding. Platt:Syneos Health: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal